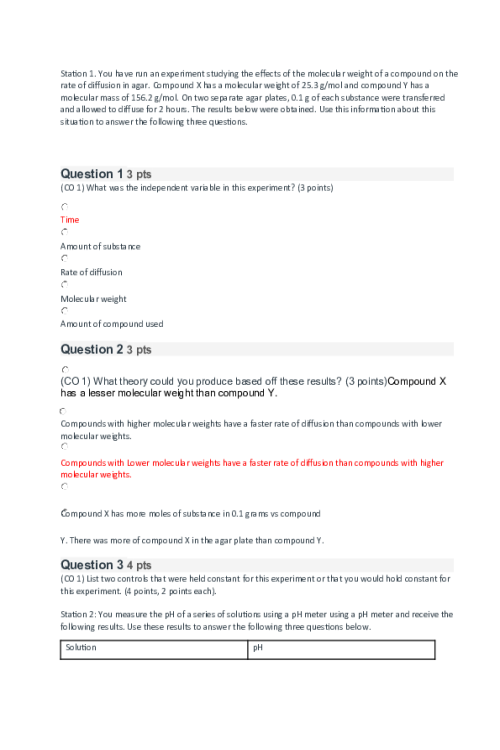
CHEM 120 Week 7 Concepts; Biochemistry
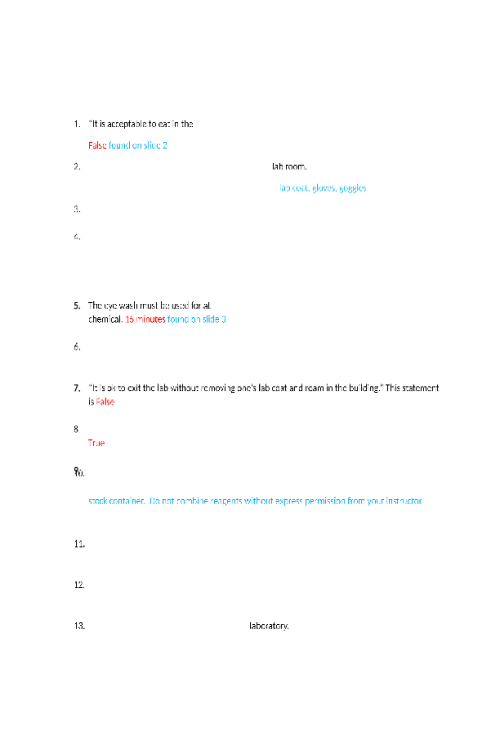
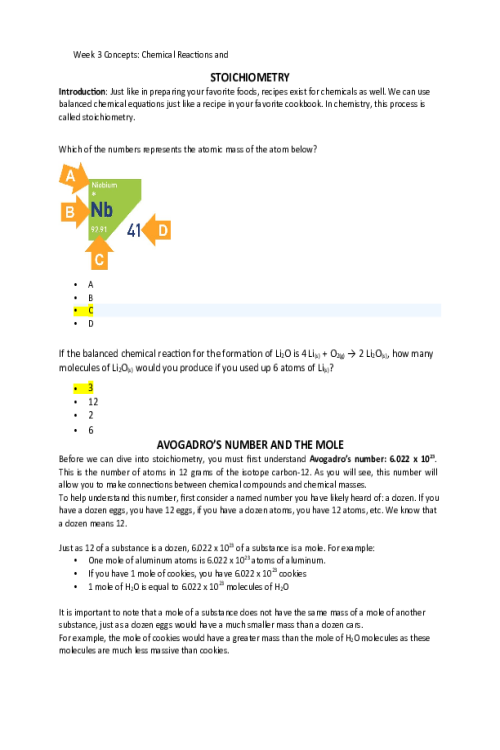
LIPIDS 524648020058In this concept, we will discuss one class of biological polymers called lipids or fats. You might be familiar with this class of biopolymers, which are found in butters and oils. We will discuss saturated and unsaturated fats, and what those terms mean for the molecule's structure as well as its properties at room temperature. Which of the following is a source of lipids? Sugar, fruits, and bread Water Steaks Butter and oils STRUCTURES AND PROPERTIES OF LIPIDS The category of biomolecules known as lipids contain fats and oils as well as fatty acids. The monomer of a lipid is a fatty acid. The fatty acids are the simplest lipids and are found as components in more complex lipids. A fatty acid contains a long carbon chain attached to a carboxylic acid group at one end. Fatty acids that contain only single bonds between carbon atoms are saturated fatty acids. Recall from the organic chemistry lesson that saturated carbon chains contain all single bonds and are called alkanes. One example of a saturated fat is butanoic acid, a component of butter. This contains a four-carbon chain with a carboxylic acid functional group on the end carbon: CH3CH2CH2COOH. If there is one double bond between carbon atoms in a fatty acid, then it is a monounsaturated fatty acid; if there are more than one double bonds between carbon atoms, then the fatty acid is polyunsaturated. You will notice in the structures of oleic acid, CH3(CH2)7CHCH(CH2)7COOH, and linoleic acid, CH3(CH2)4CHCHCH2CHCH(CH2)7COOH, the double bonds cause the molecules to curve and take up more area than a saturated fat. This decrease in the molecule’s density causes unsaturated fats to be liquid at room temperature (oils), compared to the densely packed and solid saturated fats that contain single bonds. Mnemonic: “Solid, single bonds, and saturated.” This refers to the structure (all single bonds) and relative density (solid) of the saturated fats. Mnemonic: “Unsaturated oils” The oils include unsaturated alkenes, either monounsaturated or polyunsaturated. One type of unsaturated fat is called trans fats, referring to the position of the carbons around the double bond that creates a linear structure rather than a curved structure. Trans fats are often created through the process of hydrogenation and include the hydrogenated oils found in margarine, such as trans-oleic acid. Because of their arrangement, they are similar in structure to saturated fats and thus are solids at room temperature. 101042494070 SATURATED OR UNSATURATED Lipids UNSATURATED SATURATED CH3CHCHCH2CHCHCH2COOH CH3CH2CH2CH2CH2COOH CH2CH2CH2COOH CH3CH2CH2CHCHCHCHCH2CH2COOH Answer the following questions about lipids Triglycerides are: Always unsaturated Made of fatty acid monomers Used for catalysis in the body Soluble in water Select all that apply. Saturated fatty acids containfunctional groups Alkanes Amine Carboxylic acid Halogen Alkenes From the condensed structural formula, determine if the structure represents a saturated fatty acid, monounsaturated fatty acid, or a polyunsaturated fatty acid. SATURATED FATTY ACID MONOUNSATURATED FATTY ACID POLYUNSATURATED FATTY ACID CH3CH2CHCHCH2COOH X CH3CHCHCH2CH2COOH X CH3CHCHCH2CHCHCH2COOH CH3CH2CH2CH2CH2COOH X Contains carboxylic acid = Both Saturated and Unsaturated Contains a single double carbon-to-carbon bond = Unsaturated Contains multiple double carbon-to-carbon bonds = Unsaturated Solid at room temperature = Saturated Contains no carbon-to-carbon double bonds = Saturated Liquid at room temp = Unsaturated Does not mix with water = Both Saturated and Unsaturated Both saturated and unsaturated do not mix with water. Saturated contains no double carbon-to-carbon bonds and is solid at room temperature. Unsaturated contains a single double carbon-to-carbon bond (monounsaturated) or multiple double carbon-to-carbon bonds (polyunsaturated) and are mostly liquid at room temperature. TRIGLYCERIDES, GLYCEROPHOSPHOLIPIDS, AND STEROID Fats and oils consist of fatty acids that have been bound to glycerol through an ester bond. Lipids, or fats and oils, are also found in abundance in our kitchens. While the carboxylic acid group is soluble in water, the long carbon chain makes the fatty acid insoluble in water. You can observe this by pouring equal amounts of water and vegetable or olive oil into a small, clear container. Two layers will appear: one is the polar (hydrophilic) water, one is the nonpolar (hydrophobic) oil. Because of the differences in hydrophobicity, polar and nonpolar molecules will not mix. This idea is commonly referred to as “Like Dissolves Like,” meaning polar (hydrophilic) molecules will only mix with other polar (hydrophilic) molecules; nonpolar (hydrophobic) molecules will only mix with other nonpolar molecules. Triacylglycerols, or triglycerides, are triesters of glycerol (a trihydroxy alcohol) and fatty acids. Basically, the glycerol molecule has three tails of fatty acids. These molecules are formed by esterification, the condensation of the carboxylic acid on the fatty acid and the alcohol groups on the glycerol. Triacylglycerols also undergo hydrogenation, hydrolysis, and saponification (soap-making) reactions. Glycerophospholipids are similar to triacylglycerols, except that one hydroxyl group of glycerol has been replaced by the ester of phosphoric acid and an amino alcohol and bonded through a phosphodiester bond. One consequence of their structure is that glycerophospholipids have both polar and nonpolar regions. These macromolecules are the most abundant lipid in cell membranes. Cell Membrane lipid bilayer with receptors Steroids are compounds that consist of three cyclohexane rings and one cyclopentane ring that are fused together. This structure is referred to as the steroid nucleus. A very common steroid is cholesterol, which is a component of cellular membranes, myelin sheath tissue, and brain and nerve tissue. Cholesterol is also used to synthesize Vitamin D and steroid hormones. FATTY ACIDS ATTACHING TO GLYCEROL 4427742101388Using the molecule below, answer the following questions This molecule is best described as A polyunsaturated fatty acid An unsaturated fatty acid A triglyceride A fatty acid How many fatty acids were used to create this triacylglycerol molecule? One Two Three Four The fatty acids used to create this triacylglycerol are All unsaturated All saturated All connected through an ester linkage All identical Match the lipid to the function Glycerophospholipids compromises the majority of cell membranes because it contains a polar head and a nonpolar tail Triacylglycerol used in soap making Cholesterol structure included three six-member rings fused with a five-member rings, and in cell membranes provides stability and is a precursor for vitamin D Select all that apply. Which of the following are typically solids at room temperature? Trans lipids Saturated lipids Unsaturated lipids Monosaturated lipids Polysaturated lipids Which class of lipids is found in cell membranes? Sterols Triacylglycerol Glycerophospholipid Steroids and triacylglycerol Steroids and glycerophospholipids What type of linkage is found in triacylglycerol’s? Amide Ether Peptide Ester Glycoside Triacylglycerols contain. 3 molecules of glycerol and 1 steroid 1 molecule of glycerol and 3 molecules of fatty acid 1 molecule of glycerol and 3 steroids 3 molecules of glycerol and 1 molecule of fatty acid Which of the following is true for both saturated and unsaturated fatty acids? Both are considered alkenes Both are solid at room temperature Both contain ester linkages Both are soluble in water Both contain carboxylic acid groups PROTEINS In this concept we discuss the third class of macromolecules: the proteins. Proteins are essential for multiple functions within cells: catalysis, structure, transport, energy, and many others. We will discuss the role of the amide (peptide) linkage, four levels of protein structures, and properties of the proteins. Rank the levels of protein structure from top (least complex/ structured) to bottom (most complex/ structured) Primary – sequence of amino acids Secondary – alpha helices, beta strands, and loops Tertiary – the three-dimensional structure Quaternary – interaction of multiple tertiary structures Examples of proteins include Meat and muscle Sugars and candy Butter and oil All of these STRUCTURE OF AMINO ACIDS As with our other biomolecules, to understand proteins, we first need to understand their parts. Proteins are polymers of amino acids. Amino acids have two functional groups: an amino group (-NH2) and a carboxylic acid group (-COOH). This is where their name comes from: amino acid. For all amino acids found in proteins, the amino group, the carboxylic acid group, and a hydrogen atom are bonded to the central carbon atom (called the alpha carbon). The R is the placeholder for the sidechain. Different amino acids have different side chains, but they all have the amine group, alpha carbon, and carboxylic acid groups. While there are many amino acids, only 20 different amino acids are found in humans. On the general structure of an amino acid below, click on the following parts: amino group, carboxylic acid group, and alpha hydrogen. General structure of an amino acid can be written as NH2CHRCOOH. The backbone is the portion that repeats in every amino acid: the amine, the alpha carbon, and the carboxylic acid. To show a specific amino acid, the R is replaced by the sidechain. One of the twenty amino acids is shown below. 3036961123777 As we can see, we have replaced
Related Products
CHEM 120 Week 1 Discussion Option 1; Scientific Method and Atomic Structure
Contributor: Ellyse Perry
$10
CHEM 120 Week 1 Discussion Option 2; Scientific Method and Atomic Structure
Contributor: Ellyse Perry
$10
Related Products
CHEM 120 Week 1 Discussion Option 1; Scientific Method & Atomic Structure
Contributor: Ellyse Perry
$10
CHEM 120 Week 2 Concepts; Understanding the Structure and Naming of Molecules
Contributor: Ellyse Perry
$20
CHEM 120 Week 2 Discussion; Atomic Structure - Methyl Salicylate C8H8O3
Contributor: Ellyse Perry
$10
CHEM 120 Week 5 Discussion; Organic Chemistry - Dichloro-diphenyl-trichloroethane
Contributor: Ellyse Perry
$10
CHEM 120 Week 4 Discussion; Chemistry in the World Around Us - Shampoo Solution
Contributor: Ellyse Perry
$10
CHEM 120 Week 4 Discussion; Chemistry in the World Around Us - Vinegar that contains acetic acid
Contributor: Ellyse Perry
$10
CHEM 120 Week 7 Assignment; Group Project - Stoichiometry & Dosage Calculation (CO 4)
Contributor: Ellyse Perry
$20
CHEM 120 Week 6 Discussion Option 1; Radiation; Radioactive Isotope; Cobalt-60
Contributor: Ellyse Perry
$10
CHEM 120 Week 6 Concepts; Nuclear Chemistry, Energy, and Biochemistry Part 1
Contributor: Ellyse Perry
$20
CHEM 120 Week 6 Discussion Option 2; Radiation - Internal Radiation Treatment; known as Brachytherapy
Contributor: Ellyse Perry
$10
CHEM 120 Week 7 Discussion; Flow of Genetic Information - Controversial Genetic Technology Known as Sex Selection
Contributor: Ellyse Perry
$10
CHEM 120 Week 7 Discussion; Flow of Genetic Information - Genetics in Forensic Science
Contributor: Ellyse Perry
$10
CHEM 120 Week 7 Assignment; Group Project; How vitamins and mineralstrace elements affect healthhuman body (CO 3)
Contributor: Ellyse Perry
$20
CHEM 120 Week 7 Assignment; Group Project; Corrosion of Plumbing, Erosion of Confidence
Contributor: Ellyse Perry
$20
CHEM 120 Week 8 Discussion; Wrap-Up (Health effects of Radiation on the Human Body)
Contributor: Ellyse Perry
$15