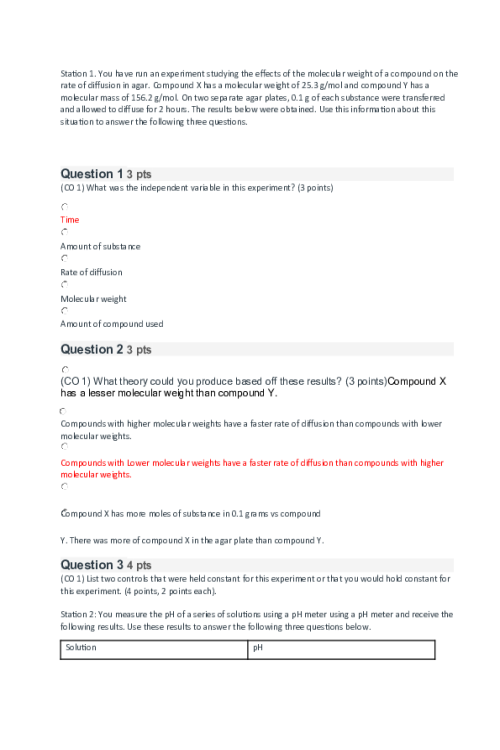
CHEM 120 Week 6 Concepts; Nuclear Chemistry, Energy, and Biochemistry Part 1
57111908209280LAWS OF THERMODYNAMICS 495554022525In this section, we explore the rules of energy that we observe in the universe. We see many examples of these laws in action on a day-to- day basis. You will learn how to compare and contrast exothermic and endothermic reactions as well as the first and second laws of thermodynamics. Which of these would you classify as an exothermic process? Evaporation of alcohol Photosynthesis Wood burning Ice melting Which one of these processes would NOT be possible? Energy is transferred in a chemical reaction Energy is created in a chemical reaction Two liquid chemicals are mixed together, and the temperature of the mixture rapidly cools Energy spontaneously flows from a hot object Energy cannot be created or destroyed in a chemical reaction. ENERGY AND FIRST LAW OF THERMODYNAMICS Energy takes many forms. A few of the more common forms of energy we observe are light, heat, mechanical energy, and electricity. Energy is the driving force of this universe and necessary for all life, light, and movement. Energy can be measured using various units, including calories and joules. A good starting point in understanding the flow of energy is the First Law of Thermodynamics: Energy in an isolated system cannot be created or destroyed, only transferred. This law is often also known as Conservation of Energy, as this law tells us that energy is conserved and simply moved from one place to another and one form to another. For example, when you drive a car down the road, you are converting the chemical energy found in gasoline into heat and kinetic (movement) energy. None of this energy is destroyed or eliminated in this process. As another example of the first law of thermodynamics in action consider a falling stone hitting the earth. The kinetic energy of this stone is converted mainly into sound, and vibration. In both of these examples, we see that energy can move from one form to another. You may have noticed the term “isolated system” in the 1st Law of Thermodynamics. This term means a system where no matter and no energy go into or out of the system. Our universe, as we understand it, is an example of such a system, so this law applies to our universe. SECOND LAW OF THERMODYNAMICS The second law of thermodynamics tells us about how energy in the universe behaves in terms of flow and organization. The amount of entropy in an isolated system irreversibly increases over time. This law tells us that the amount of entropy, or disorder, in the universe is constantly increasing. An important reason for this is that heat spontaneously and irreversibly transfers from a hot body to a cold body. An example that illustrates both of these points is a cup of hot coffee sitting outside on a cold winter's day. At first, the heat is localized into the area of the liquid in the cup; however, the heat quickly begins to disperse into the surrounding environment spontaneously. Over time, the coffee in the cup will have the same temperature as the surrounding environment as the temperatures even out. In this way, we see that: Heat was spontaneously and irreversibly transferred from a higher temperature system area to the lower temperature surroundings. Disorder increased as the localized heat spread through the surroundings. For each of the situations, determine if the observed behavior is a consequence of the first or second Law of Thermodynamics: Situation Select Law As a rocket takes off, energy in the fuel is converted into kinetic energy, heat, and light. 1st Law of Thermodynamics An ice cube melts on a hot day. 2nd Law of Thermodynamics On a hot day, you turn on the oven, causing the room to become even hotter. 2nd Law of Thermodynamics Turning on a computer results in electricity being converted into mechanical work, heat, and light. 1st Law of Thermodynamics ENDOTHERMIC AND EXOTHERMIC REACTIONS 476757953005Energy can flow into a system from the surroundings or from the surroundings into the system. The terminology we use for these processes is: Exothermic: Energy from system to surroundings (energy released) Endothermic: Energy from surroundings to system (Energy absorbed) The image illustrates this process. We often measure this energy in the form of heat, so heat flowing from the system to the surroundings is considered exothermic, while heat flowing into the system is considered endothermic. A good mnemonic is that exo = exit (energy exits). An example of an exothermic process is a burning match. The burning match releases heat into the surroundings and is thus classified as exothermic. On the other hand, you may have used a chemical cooling pack to treat an injury. In a chemical cooling pack, the chemical reaction absorbs heat from the surroundings, cooling your injury. Since energy is going from the surroundings to the system, we would consider this process endothermic. What about ice melting? Would you consider this to be endothermic or exothermic? Is energy going in, or is energy going out? Ice melting is endothermic as energy goes into the ice, giving the molecules of water the energy they need to move more quickly. This transforms the solid. As a material absorbs energy (endothermic), the atoms and molecules in the material move more quickly and the state of the material changes from solid to liquid to gas. Opposingly, as a material releases energy (exothermic), the atoms and molecules slow down, moving from gas to liquid to solid. Match the reaction to the type: Endothermic Photosynthesis Chemical ice packs Methanol evaporating Iron melting o Exothermic Water freezing Burning gasoline Water condensing on a cool surface Two chemicals are mixed together, producing heat When a cool penny is placed in a hot car in the summer, the heat flows spontaneously from the interior of the car to the penny in an illustration of the 2nd Law of thermodynamics. Sort the following process as exothermic or endothermic: Endothermic: A pair of chemicals are mixed together in a beaker and the beaker becomes cold Carbon dioxide converts from a solid to a gas o Exothermic: Your body digests food for energy, generating excess heat On a humid day water condenses on a cool surface Wood burns in a bonfire When sodium hydroxide is dissolved in water within a beaker, you observe that the beaker becomes warm. This situation illustrates: The 2nd Law of Thermodynamics The 1st Law of Thermodynamics An Exothermic reaction Select all the following situations that you would classify as endothermic processes: Ammonium nitrate dissolves in water and the solution becomes cold Lava cools and becomes a solid Butane combusts to give a flame in a lighter Hand sanitizer evaporating RADIATION When we hear the word radiation, often our minds turn to power plants, nuclear weapons, and reactors. In fact, radiation is so much more and has been part of our universe from the very beginning. In this lesson, we will explore the concept of radiation and the types of radiation that we encounter. Which of the following are types of electromagnetic radiation? X-rays Alpha particles Light Radio waves Which type of radiation would you consider ionizing radiation? Visible light Microwaves Gamma rays Sound waves Which of the following procedures in healthcare involve the use of radiation to take images? Temperature measurements using thermometers Pulse measurements CT scans X-Ray Radiation is a broad term that refers to energy transferred over distance as rays, particles, or waves. Radiation has always been a part of our world. By the end of this lesson, you should no longer think of all radiation as dangerous, as there are many forms of radiation with a wide range of properties. The main categories of radiation we will be focusing on are: Particulate radiation: Energy transmitted through small particles Electromagnetic radiation: Energy transmitted through waves or rays without mass Particulate radiation includes alpha particles as well as electron-based transmission, known as beta particle radiation. These types of radiation have mass, even if that mass is incredibly small. You will learn more about the properties of particulate radiation at another time. Electromagnetic radiation has no mass and includes X-Rays, all forms of light, radio waves, microwaves, and all other sources of radiation without mass. As you see from the examples, you are always being exposed to electromagnetic radiation any time there is any degree of light in your presence. Electromagnetic radiation can also be said to exist as massless particles of energy known as photons and exist simultaneously as particles and waves. IONIZING AND NON-IONIZING RADIATION Another way to categorize radiation is as ionizing or non-ionizing radiation. As you may recall, ions are charged atoms or molecules. Ionizing radiation is radiation that is able to cause atoms or molecules to become charged by removing electrons. Non-ionizing radiation is NOT able to cause the formation of ions as types of radiation that fall into this category and cannot remove electrons from atoms or molecules. The reason that some radiation is ionizing, and others are non-ionizing comes down to energy. High energy radiation is better able to cause ionization than lower energy forms. This is because it takes energy to remove electrons. These categories of radiation overlap with the other categories of radiation: particulate and electromagnetic. As an example, visible light is a type of electromagnetic radiation that is non-ionizing while X-Rays are a type of electromagnetic radiation that is ionizing. RADIATION ALL AROUND US 489965974595Now that we understand the major classes of radiation, let us look into specific examples in the real world. Radiation appears in many forms, and before moving on, take a moment to identify some forms of radiation that you may be exposed to right now. Once you have recorded the types of radiation you propose you are being exposed to, keep this on hand as we explore real world sources of radiation. Keep in mind, this is not an exhaustive list, but will give us the ability to identify radiation we encounter on a day-to-day basis. LIGHT As previously mentioned, all light is a form of radiation. Light exists as both a particle and a wave and, like all other sources of electromagnetic radiation, consists of photons. The light that we see, known as visible light, is only part of the light that we are exposed to. Light exists as a spectrum of wavelengths with shorter wavelengths being higher in energy. On the long wavelength end, we have radio waves, cell phone waves, microwaves, and infrared radiation. These are all non-ionizing. Next, we have visible light, which is the type of light that we are able to see as humans. This radiation is not ionizing. Higher in energy than visible light, with have ultraviolet (UV light), followed by X-rays and gamma rays. At the high end of the UV scale and beyond, this radiation is ionizing. The spectrum above illustrates the electromagnetic spectra of light. This light is emitted from a variety of sources with examples given below: Source Electromagnetic sources released Sun Entire spectra Incandescent bulbs Visible and partially into infrared White LED Visible Tanning bed bulbs Ultra violet Microwave ovens Microwaves Is the type of radiation ionizing or non-ionizing? Ionizing: X-rays Gamma Rays o Non-ionizing Microwaves Infrared Radio Waves Visible Light The field of healthcare depends on ionizing
Related Products
CHEM 120 Week 1 Discussion Option 1; Scientific Method and Atomic Structure
Contributor: Ellyse Perry
$10
CHEM 120 Week 1 Discussion Option 2; Scientific Method and Atomic Structure
Contributor: Ellyse Perry
$10
Related Products
CHEM 120 Week 1 Discussion Option 1; Scientific Method & Atomic Structure
Contributor: Ellyse Perry
$10
CHEM 120 Week 2 Concepts; Understanding the Structure and Naming of Molecules
Contributor: Ellyse Perry
$20
CHEM 120 Week 2 Discussion; Atomic Structure - Methyl Salicylate C8H8O3
Contributor: Ellyse Perry
$10
CHEM 120 Week 5 Discussion; Organic Chemistry - Dichloro-diphenyl-trichloroethane
Contributor: Ellyse Perry
$10
CHEM 120 Week 4 Discussion; Chemistry in the World Around Us - Shampoo Solution
Contributor: Ellyse Perry
$10
CHEM 120 Week 4 Discussion; Chemistry in the World Around Us - Vinegar that contains acetic acid
Contributor: Ellyse Perry
$10
CHEM 120 Week 7 Assignment; Group Project - Stoichiometry & Dosage Calculation (CO 4)
Contributor: Ellyse Perry
$20
CHEM 120 Week 6 Discussion Option 1; Radiation; Radioactive Isotope; Cobalt-60
Contributor: Ellyse Perry
$10
CHEM 120 Week 6 Discussion Option 2; Radiation - Internal Radiation Treatment; known as Brachytherapy
Contributor: Ellyse Perry
$10
CHEM 120 Week 7 Discussion; Flow of Genetic Information - Controversial Genetic Technology Known as Sex Selection
Contributor: Ellyse Perry
$10
CHEM 120 Week 7 Discussion; Flow of Genetic Information - Genetics in Forensic Science
Contributor: Ellyse Perry
$10
CHEM 120 Week 7 Assignment; Group Project; How vitamins and mineralstrace elements affect healthhuman body (CO 3)
Contributor: Ellyse Perry
$20
CHEM 120 Week 7 Assignment; Group Project; Corrosion of Plumbing, Erosion of Confidence
Contributor: Ellyse Perry
$20
CHEM 120 Week 8 Discussion; Wrap-Up (Health effects of Radiation on the Human Body)
Contributor: Ellyse Perry
$15