CHEM 120 Week 5 Concepts; Redox and Organic Chemistry
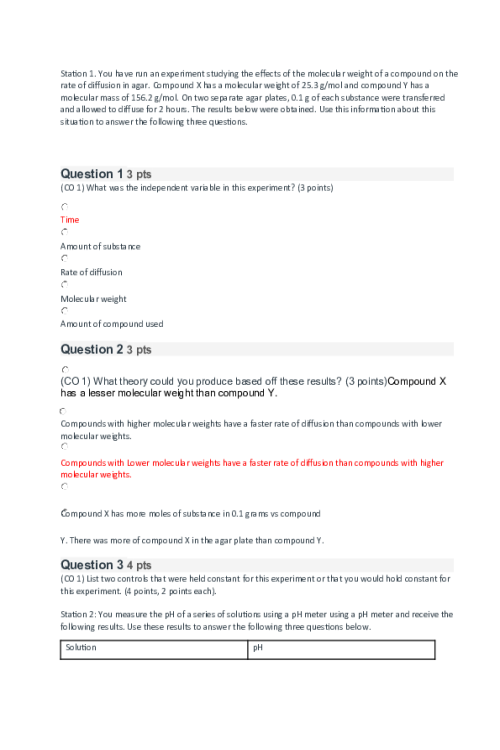
OXIDATION-REDUCTION Reduction and oxidation are intertwined concepts that are important to understanding some of the most important chemical reactions in the human body. In this lesson, we will explore the relationship between these terms and the how to identify what chemicals in a reduction/oxidation reaction (redox reaction) are playing which parts. Redox reactions involve a transfer of: Nucleons Neutrons Electrons Protons If iron reacts with oxygen gas in the reaction2 Fe(s) + O2(g) →2Fe O(s) , what is oxidized in this reaction? No oxidation is occurring here FeO O2 Fe REDUCTION AND OXIDATION Reduction and oxidation involves the transfer of electrons between two species within a chemical reaction. Not all chemical reactions involve reduction and oxidation; however, the reactions that involve reduction and oxidation are called Redox reactions. As usual, we need to understand a bit of terminology in order to understand these reactions further. Oxidation: loss of electrons Reduction: gain of electrons You may find the word “reduction” a bit of an odd choice for a reaction that involves gaining of electrons. The key is to remember that electrons have a -1 charge, and thus, the more electrons an atom gains, the more negative the charge. To help remember these terms, the mnemonic below can help: LEO the lion goes GER: Loss of Electrons is Oxidation and Gain of Electrons is Reduction In a chemical reaction, oxidation cannot happen without reduction and reduction cannot happen without oxidation. Oxidation involves the loss of electrons while reduction involves the gain of electrons. Consider a reaction where a Li+ is converted into Li. Do you think that this Lithium atom gained or lost an electron? gained electron lost electron IDENTIFYING OXIDATION AND REDUCTION We know that electrons have a -1 charge, and we understand that +1 -1 = 0. So, if Li+ gains an electron, this is what cancels out the positive (+1) charge and makes this atom neutral. 5845809269539The rule for oxidation and reduction, based on the gain or loss of electrons is that: As the charge of a species increases in the positive direction, the species is oxidized. As the charge of a species decreases, becoming more negative, the species is reduced. A visualization of this relationship appears in the image. Note the direction of the arrows. When the Li+ became Li, we see that we moved from +1 to 0, or downwards, telling us that this process was reduction. As we see, if charges are present, determining reduction and oxidation is as easy as looking at how the charges change. In the following reactions, indicate if the species is oxidized or reduced: Mg2+ becomes Mg Reduced becomes O2 Reduced Fe2+ becomes Fe3+ Oxidized F becomes F- Reduced Cu becomes Cu+ Oxidized H becomes H+ Oxidized OXIDATION AND REDUCING AGENTS Reduction cannot happen without oxidation and oxidation cannot happen without reduction. For example, when a species is oxidized, losing one or more electrons, those electrons must go to another species in the reaction, causing the other species to be reduced. Oxidizing agents are reduced in a chemical reaction (because oxidizing agents accept electrons) 1828800639616Reducing agents are oxidized in a chemical reaction (because reducing agents donate electrons) As seen in theimage, species A transfers electrons to species B, and thus species A is the reducing agent. You also see that since species A has lost electrons, species A is oxidized. In other words, the species being oxidized is the reducing agent while the species being reduced is the oxidizing agent (species B in this case). In chemical reactions, a common oxidizing agent is oxygen gas, O2. For example, rust forms when O2 reacts with iron, Fe, causing oxidation. 2 Fe(s) + O2(g) → FeO(S) In this reaction, we would consider the O2 to be the oxidizing agent and Fe to be the reducing agent, as electrons were transferred from Fe to O. A common reducing agent is hydrogen gas, H2. In the reaction below, we see how hydrogen gas can reduce the iron, forming Fe from FeO. FeO(S) + H2(g) → Fe +H2O(g) In this reaction, we would consider H2 to be the reducing agent and FeO to be the oxidizing agent as electrons were transferred from the H2 to the Fe in the FeO. Putting together what you have learned in this lesson, you can identify oxidizing and reducing agents. The key to this is to remember: Oxidizing agents are reduced in a chemical reaction (because oxidizing agents accept electrons) R
Related Products
CHEM 120 Week 1 Discussion Option 1; Scientific Method and Atomic Structure
Contributor: Ellyse Perry
$10
CHEM 120 Week 1 Discussion Option 2; Scientific Method and Atomic Structure
Contributor: Ellyse Perry
$10
Related Products
CHEM 120 Week 1 Discussion Option 1; Scientific Method & Atomic Structure
Contributor: Ellyse Perry
$10
CHEM 120 Week 2 Concepts; Understanding the Structure and Naming of Molecules
Contributor: Ellyse Perry
$20
CHEM 120 Week 2 Discussion; Atomic Structure - Methyl Salicylate C8H8O3
Contributor: Ellyse Perry
$10
CHEM 120 Week 5 Discussion; Organic Chemistry - Dichloro-diphenyl-trichloroethane
Contributor: Ellyse Perry
$10
CHEM 120 Week 4 Discussion; Chemistry in the World Around Us - Shampoo Solution
Contributor: Ellyse Perry
$10
CHEM 120 Week 4 Discussion; Chemistry in the World Around Us - Vinegar that contains acetic acid
Contributor: Ellyse Perry
$10
CHEM 120 Week 7 Assignment; Group Project - Stoichiometry & Dosage Calculation (CO 4)
Contributor: Ellyse Perry
$20
CHEM 120 Week 6 Discussion Option 1; Radiation; Radioactive Isotope; Cobalt-60
Contributor: Ellyse Perry
$10
CHEM 120 Week 6 Concepts; Nuclear Chemistry, Energy, and Biochemistry Part 1
Contributor: Ellyse Perry
$20
CHEM 120 Week 6 Discussion Option 2; Radiation - Internal Radiation Treatment; known as Brachytherapy
Contributor: Ellyse Perry
$10
CHEM 120 Week 7 Discussion; Flow of Genetic Information - Controversial Genetic Technology Known as Sex Selection
Contributor: Ellyse Perry
$10
CHEM 120 Week 7 Discussion; Flow of Genetic Information - Genetics in Forensic Science
Contributor: Ellyse Perry
$10
CHEM 120 Week 7 Assignment; Group Project; How vitamins and mineralstrace elements affect healthhuman body (CO 3)
Contributor: Ellyse Perry
$20
CHEM 120 Week 7 Assignment; Group Project; Corrosion of Plumbing, Erosion of Confidence
Contributor: Ellyse Perry
$20
CHEM 120 Week 8 Discussion; Wrap-Up (Health effects of Radiation on the Human Body)
Contributor: Ellyse Perry
$15