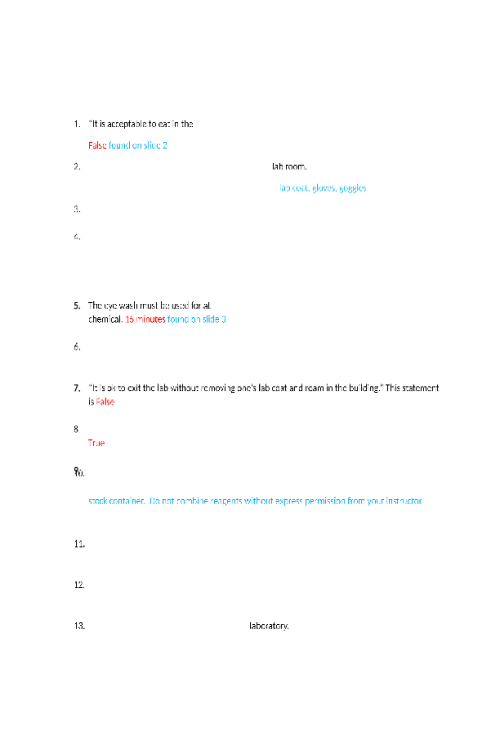
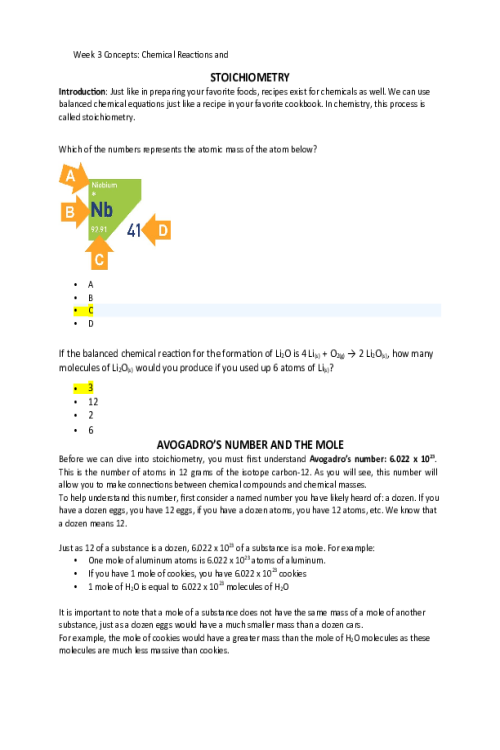
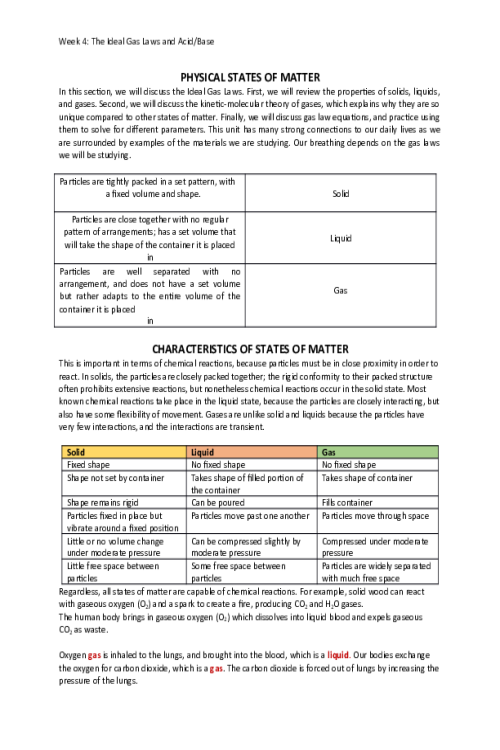
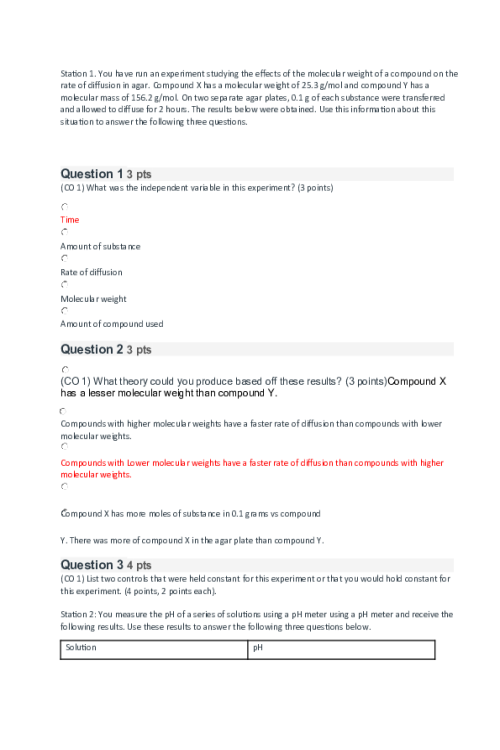
CHEM 120 Week 1 Concepts; Atomic Structure
ATOMIC STRUCTURE In many ways, this lesson is the start of your journey into chemistry. In this foundational lesson, we will learn about the building blocks of matter: atoms. Alchemists, the precursors to chemists, had a saying: “As above, so below”. A modern interpretation of this saying is that the properties of what we can see can help us understand the properties of what we cannot. In this lesson, we will learn how the properties of atoms determine the properties of the world around us. MATTER 4572000112886Look around you and note any object that you could touch. Each of those objects is made of matter. In fact matter is anything that has both mass and volume, in other words, anything that we could measure the mass of (no matter how small that mass may be) that also takes up space. For example, consider a flower, say a daisy. If I were to put the daisy on a scale, it would have a mass. The flower would also occupy a measurable amount of space, meaning it has volume. As the daisy would have both mass and volume, it would be considered matter. On the other hand, the image of that daisy would not be considered matter as the image has no mass, and therefore, is not considered to be matter. Instead, the image is made of energy. If power were to suddenly stop flowing to your screen, you would no longer see the image as the light hitting your eye would no longer be coming from the screen. We can also see that the matter surrounding us is not all the same. Water has very different properties from plastic which, in turn, has different properties from wood. As we see here, the term matter is a very broad term, and we can sort matter into different categories; however, to do this, we must first look at the building blocks of our universe: atoms. ELEMENTS and ATOMS The world around and within us consists of atoms. Everything that we touch or feel is made out of these basic building blocks. But what exactly is an atom? To answer this question, we first must understand what the term “element” means. An element is a form of matter that cannot be broken down any further by chemical reactions. Examples of elements include iron, hydrogen, and helium. Each element has specific properties.
Related Products
CHEM 120 Week 1 Discussion Option 1; Scientific Method and Atomic Structure
Contributor: Ellyse Perry
$10
CHEM 120 Week 1 Discussion Option 2; Scientific Method and Atomic Structure
Contributor: Ellyse Perry
$10
CHEM 120 Week 1 Discussion Option 1; Scientific Method & Atomic Structure
Contributor: Ellyse Perry
$10
Related Products
CHEM 120 Week 2 Concepts; Understanding the Structure and Naming of Molecules
Contributor: Ellyse Perry
$20
CHEM 120 Week 2 Discussion; Atomic Structure - Methyl Salicylate C8H8O3
Contributor: Ellyse Perry
$10
CHEM 120 Week 5 Discussion; Organic Chemistry - Dichloro-diphenyl-trichloroethane
Contributor: Ellyse Perry
$10
CHEM 120 Week 4 Discussion; Chemistry in the World Around Us - Shampoo Solution
Contributor: Ellyse Perry
$10
CHEM 120 Week 4 Discussion; Chemistry in the World Around Us - Vinegar that contains acetic acid
Contributor: Ellyse Perry
$10
CHEM 120 Week 7 Assignment; Group Project - Stoichiometry & Dosage Calculation (CO 4)
Contributor: Ellyse Perry
$20
CHEM 120 Week 6 Discussion Option 1; Radiation; Radioactive Isotope; Cobalt-60
Contributor: Ellyse Perry
$10
CHEM 120 Week 6 Concepts; Nuclear Chemistry, Energy, and Biochemistry Part 1
Contributor: Ellyse Perry
$20
CHEM 120 Week 6 Discussion Option 2; Radiation - Internal Radiation Treatment; known as Brachytherapy
Contributor: Ellyse Perry
$10
CHEM 120 Week 7 Discussion; Flow of Genetic Information - Controversial Genetic Technology Known as Sex Selection
Contributor: Ellyse Perry
$10
CHEM 120 Week 7 Discussion; Flow of Genetic Information - Genetics in Forensic Science
Contributor: Ellyse Perry
$10
CHEM 120 Week 7 Assignment; Group Project; How vitamins and mineralstrace elements affect healthhuman body (CO 3)
Contributor: Ellyse Perry
$20
CHEM 120 Week 7 Assignment; Group Project; Corrosion of Plumbing, Erosion of Confidence
Contributor: Ellyse Perry
$20
CHEM 120 Week 8 Discussion; Wrap-Up (Health effects of Radiation on the Human Body)
Contributor: Ellyse Perry
$15