CHEM 120 Week 2 Concepts; Understanding the Structure and Naming of Molecules
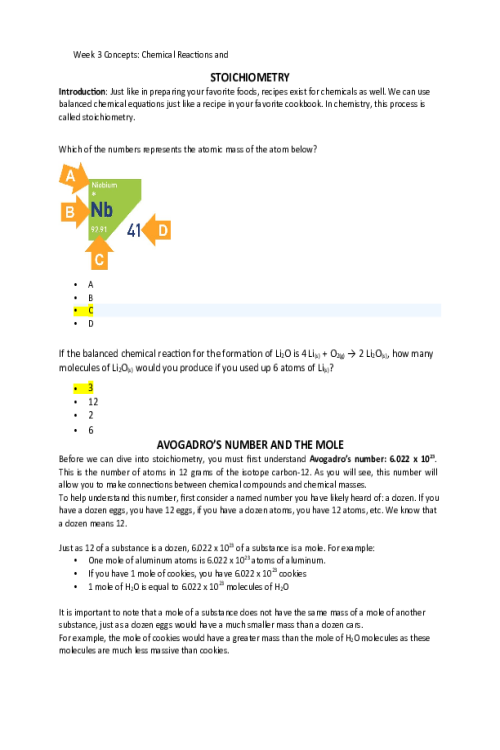
Introduction Building on the fundamentals of covalent bonding, we will be looking deeper into the structures and behaviors of molecular compounds. We will be exploring how covalent bonding is involved in the structure of these molecules and determines how they interact with the world around them. What shape would you expect the compound Hydrogen Fluoride to have? Pyramidal Tetrahedral Linear Bent Which of the following substances would you not expect to dissolve in water? Alcohol Oil Sugar Salt COVALENT MOLECULES 519684035225The way covalent molecules behave is strongly influenced by their structure. A major theme in chemistry overall is that structure greatly impacts properties. This structure/property relationships is why, for example, some liquids have a higher boiling point than others and why certain vitamins are easier to digest than others. To start out, we will be taking a deeper dive into Lewis Dot Structure andsee how we can use skill to model the covalent bonding of simple molecular compounds and determine their shapes. Next, we will look at the concept of bond polarity. Finally, we will look at how shape and polarityof these molecules gives them the properties we observe in the real world. LEWIS DOT STRUCTURE FOR MOLECULAR COMPOUNDS Lewis dot structure is a helpful tool for determining the bonding properties of a nonmetal based molecular structure. As a quick reminder, molecular compounds form from non-metals and form covalent bonds. Each of these covalent bonds is made of two electrons, and multiple bonds can be formed between a pair of atoms to make what we call double and triple bonds. Let us consider how to show the Lewis Dot Structure of the molecule water: H2O. The first question we must ask ourselves is: How many unpaired valence electrons do each of these atoms have? 50723791976955175250197695 HO 1596389-1807955255259-2582655257800-1490455144770-410954970779-175715Hydrogen atoms each havean oxygen atom has 1 unpaired valence electron.2 unpaired valance electrons. Here are the Lewis dot structures of the three atoms in this structure. Since there are only two electrons possible in a single covalent bond, the unpaired electrons are the ones that will be most able to form bonds. 51587401844615273040187001 HHO 2625089-1730544028440-1819445400040-2175045397500-1120945246370-282745092700-165434Now let us consider how we would connect these
Related Products
CHEM 120 Week 1 Discussion Option 1; Scientific Method and Atomic Structure
Contributor: Ellyse Perry
$10
CHEM 120 Week 1 Discussion Option 2; Scientific Method and Atomic Structure
Contributor: Ellyse Perry
$10
Related Products
CHEM 120 Week 1 Discussion Option 1; Scientific Method & Atomic Structure
Contributor: Ellyse Perry
$10
CHEM 120 Week 2 Discussion; Atomic Structure - Methyl Salicylate C8H8O3
Contributor: Ellyse Perry
$10
CHEM 120 Week 5 Discussion; Organic Chemistry - Dichloro-diphenyl-trichloroethane
Contributor: Ellyse Perry
$10
CHEM 120 Week 4 Discussion; Chemistry in the World Around Us - Shampoo Solution
Contributor: Ellyse Perry
$10
CHEM 120 Week 4 Discussion; Chemistry in the World Around Us - Vinegar that contains acetic acid
Contributor: Ellyse Perry
$10
CHEM 120 Week 7 Assignment; Group Project - Stoichiometry & Dosage Calculation (CO 4)
Contributor: Ellyse Perry
$20
CHEM 120 Week 6 Discussion Option 1; Radiation; Radioactive Isotope; Cobalt-60
Contributor: Ellyse Perry
$10
CHEM 120 Week 6 Concepts; Nuclear Chemistry, Energy, and Biochemistry Part 1
Contributor: Ellyse Perry
$20
CHEM 120 Week 6 Discussion Option 2; Radiation - Internal Radiation Treatment; known as Brachytherapy
Contributor: Ellyse Perry
$10
CHEM 120 Week 7 Discussion; Flow of Genetic Information - Controversial Genetic Technology Known as Sex Selection
Contributor: Ellyse Perry
$10
CHEM 120 Week 7 Discussion; Flow of Genetic Information - Genetics in Forensic Science
Contributor: Ellyse Perry
$10
CHEM 120 Week 7 Assignment; Group Project; How vitamins and mineralstrace elements affect healthhuman body (CO 3)
Contributor: Ellyse Perry
$20
CHEM 120 Week 7 Assignment; Group Project; Corrosion of Plumbing, Erosion of Confidence
Contributor: Ellyse Perry
$20
CHEM 120 Week 8 Discussion; Wrap-Up (Health effects of Radiation on the Human Body)
Contributor: Ellyse Perry
$15