Concepts; Chemical Reactions and Calculations
STOICHIOMETRY Introduction: Just like in preparing your favorite foods, recipes exist for chemicals as well. We can use balanced chemical equations just like a recipe in your favorite cookbook. In chemistry, this process is called stoichiometry.Which of the numbers represents the atomic mass of the atom below? 91440093519 A B C D If the balanced chemical reaction for the formation of Li2O is 4 Li(s) + O2(g) → 2 Li2O(s), how many molecules of Li2O(s) would you produce if you used up 6 atoms of Li(s)? • 3 12 2 6 AVOGADRO’S NUMBER AND THE MOLE Before we can dive into stoichiometry, you must first understand Avogadro’s number: 6.022 x 1023. This is the number of atoms in 12 grams of the isotope carbon-12. As you will see, this number will allow you to make connections between chemical compounds and chemical masses. To help understand this number, first consider a named number you have likely heard of: a dozen. If you have a dozen eggs, you have 12 eggs, if you have a dozen atoms, you have 12 atoms, etc. We know that a dozen means 12. Just as 12 of a substance is a dozen, 6.022 x 1023 of a substance is a mole. For example: One mole of aluminum atoms is 6.022 x 1023 atoms of aluminum. If you have 1 mole of cookies, you have 6.022 x 1023 cookies 1 mole of H2O is equal to 6.022 x 1023 molecules of H2O It is important to note that a mole of a substance does not have the same mass of a mole of another substance, just as a dozen eggs would have a much smaller mass than a dozen cars. For example, the mole of cookies would have a greater mass than the mole of H2O molecules as these molecules are much less massive than cookies. ATOMIC MASS AND MOLAR MASS 4946650121494We will now learn the relationship between moles and the atomic masses found on the periodic table. As you may recall from our earlier lessons, the periodic table gives us the atomic masses of each substance in the lower left. The un
Related Products
Greater Good Analysis; Social Technology - Key Moral Problems Created by Technology
Contributor: SecureGrades
$10
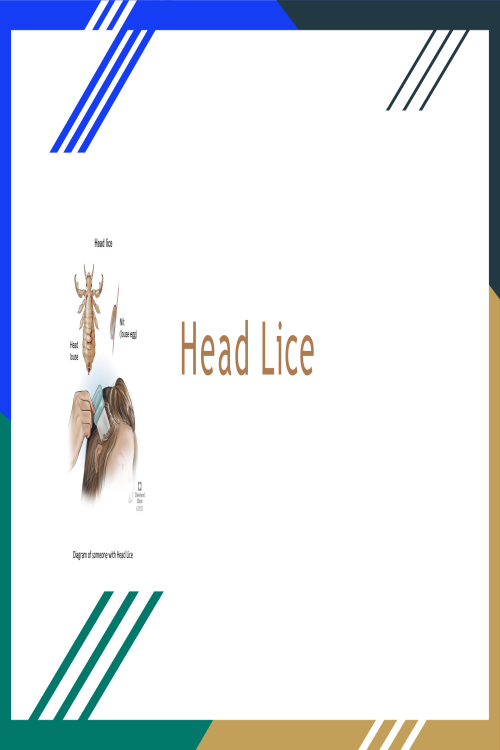
Head Lice Presentation; What Causes head Lice and What are Ways to Stop it from Spreading.pptx
Contributor: SecureGrades
$10
Importance of Nonverbal Communication in Nursing Powerpoint Presentation
Contributor: SecureGrades
$10
How Do You Collaborate Online; BENEFICIAL USES OF MICROSOFT OFFICE ONLINE COLLABORATION.pptx
Contributor: SecureGrades
$10
Related Products
Google Inc. Google LLC is an American Multinational Technology Company
Contributor: SecureGrades
$10
Painkillers Are Easy to Get Into but Hard to Escape; The Image of Addiction
Contributor: SecureGrades
$10
Philosophy of Teaching and Learning; I Believe That Everyone Can Learn
Contributor: SecureGrades
$10
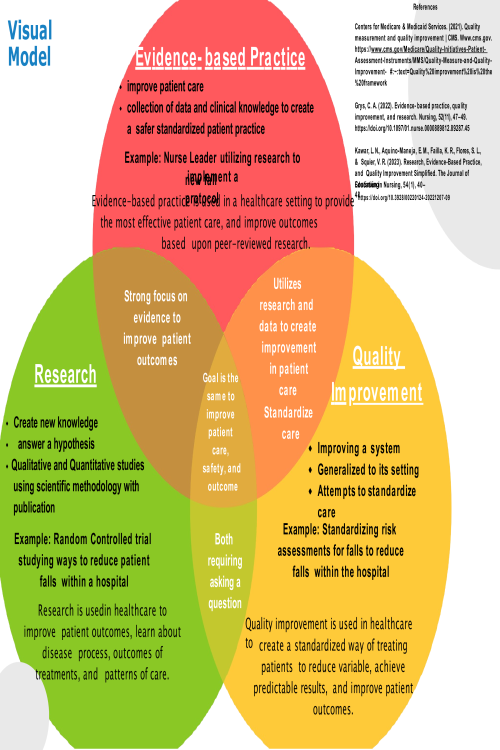
Quality Models; The Way to Get Started is to Quit Talking and Begin Doing
Contributor: SecureGrades
$10
Understanding ACEs and Trauma-Informed Care for Primary Care Providers Presentation
Contributor: SecureGrades
$10